Consider the balanced equation of aluminum reacting with sulfuric acid, a captivating chemical reaction that unveils the intricate interplay between elements and compounds. This reaction holds immense significance in various scientific and industrial applications, and delving into its intricacies will provide a deeper understanding of fundamental chemical principles.
The reaction between aluminum and sulfuric acid is a prime example of a redox reaction, where one substance undergoes oxidation while another undergoes reduction. By examining the balanced equation, we can identify the reactants, products, and stoichiometric ratios involved in this fascinating transformation.
Chemical Reaction
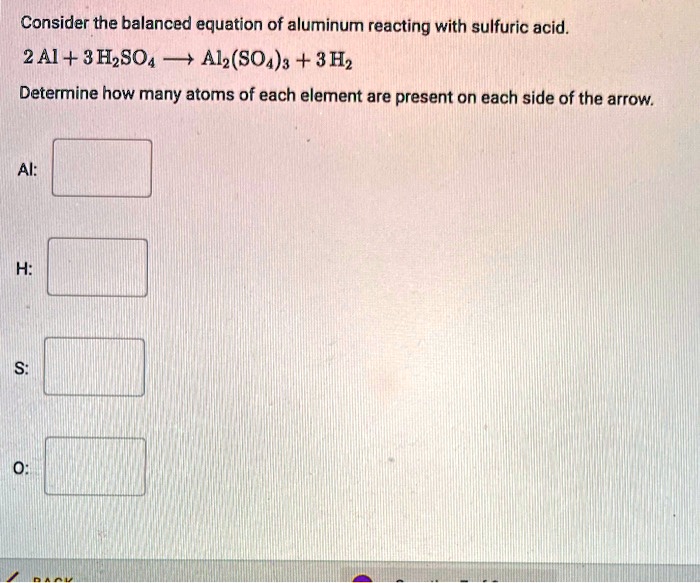
The reaction between aluminum and sulfuric acid is a redox reaction, where aluminum is oxidized and sulfuric acid is reduced. The balanced equation for the reaction is:
2Al + 3H2SO 4→ Al 2(SO 4) 3+ 3H 2
Reactants and Products
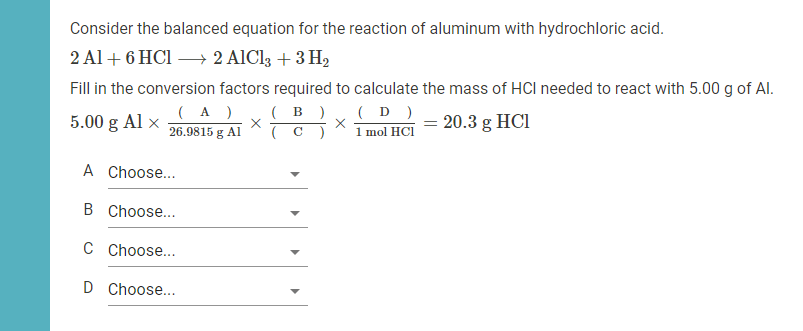
The reactants in this reaction are aluminum (Al) and sulfuric acid (H 2SO 4). Aluminum is a silvery-white metal that is highly reactive, while sulfuric acid is a corrosive liquid that is a strong acid.
The products of the reaction are aluminum sulfate (Al 2(SO 4) 3) and hydrogen gas (H 2). Aluminum sulfate is a white solid that is soluble in water, while hydrogen gas is a colorless and odorless gas that is highly flammable.
Reaction Conditions, Consider the balanced equation of aluminum reacting with sulfuric acid
The reaction between aluminum and sulfuric acid is exothermic, meaning that it releases heat. The reaction rate is increased by increasing the temperature, increasing the concentration of sulfuric acid, and adding a catalyst.
Reaction Mechanism
The reaction mechanism for the reaction between aluminum and sulfuric acid is complex and involves several steps. The first step is the formation of a complex between aluminum and sulfuric acid. This complex then undergoes a series of redox reactions to form the products of the reaction.
Applications: Consider The Balanced Equation Of Aluminum Reacting With Sulfuric Acid
The reaction between aluminum and sulfuric acid is used in a variety of applications, including:
- The production of aluminum sulfate, which is used as a coagulant in water treatment plants.
- The cleaning of metal surfaces.
- The production of hydrogen gas.
FAQs
What is the balanced equation for the reaction between aluminum and sulfuric acid?
2Al + 3H2SO4 → Al2(SO4)3 + 3H2
What are the products of the reaction?
Aluminum sulfate [Al2(SO4)3] and hydrogen gas [H2]
What is the role of aluminum in this reaction?
Aluminum acts as the reducing agent, undergoing oxidation from a neutral state to +3.
What is the role of sulfuric acid in this reaction?
Sulfuric acid acts as the oxidizing agent, undergoing reduction from +6 to +2.
